Providing minimally invasive
cryogenic solutions for safer lung
disease diagnosis and therapy
Endocision is bronchoscopy made cool.
The next generation in
transbronchial lung cryobiopsy
The best way to diagnose Interstitial Lung Disease today involves a visit
to the operating room, and carries risks of severe or fatal complications
We developed a better way.
Endocision's innovative cryobiopsy platform enables safer, effective, and
cost-effective lung biopsy and will expand access to lung disease diagnosis.
- Simplified management of complications
- No loss of positioning or visualization
- No capital equipment requirement
- Single-user procedure
- Comparable tissue samples as standard practice
- Compatible with robotic bronchoscopes
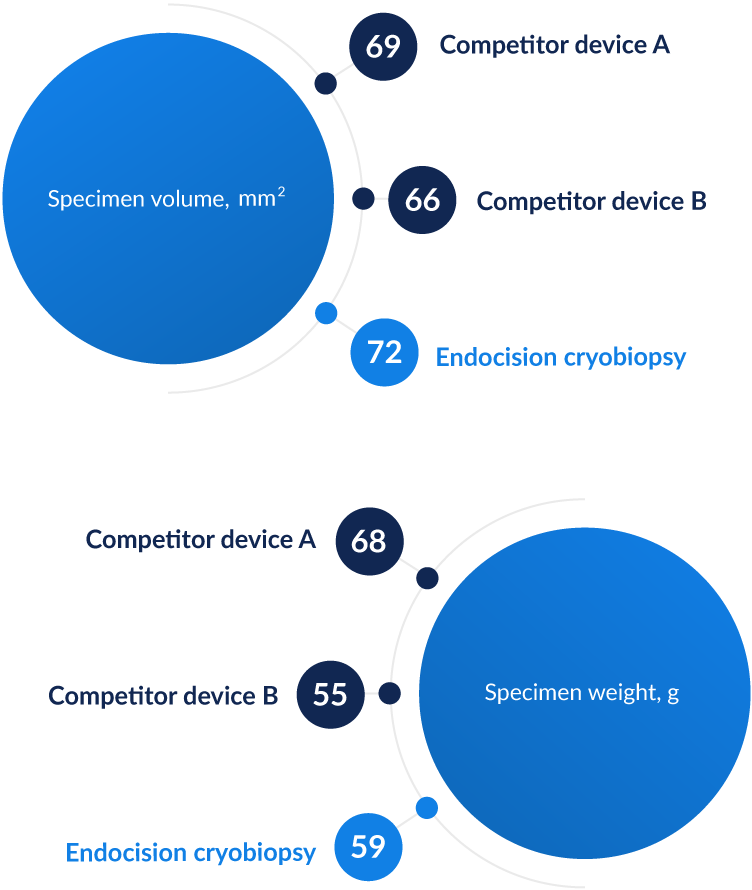
See how we compare
Our testing shows that Endocision's device produces samples
that are comparable to current standard.

Get to know us
Our Scientific Advisory Board
Careers
Want to join our team? Keep an eye out for our openings below.
There are no job listings at the moment.
















